The eBay Oils (Part 3)
Welcome back to the eBay Oils! If you missed the previous two installments, wherein we describe what we found in some old oils that Ryan bought on eBay, you can read them here and here.
 HyVis 4 Winter
HyVis 4 Winter
We’re going to kick this party off with an oil that I personally have never heard of: HyVis 4 Winter Motor Oil. The can looks very groovy and it has no zip code, so I’m placing it from the very early 1960s or late 1950s. It’s “Mileage Metered” and it has a picture of a medal with a ribbon on the can, so you know it’s good stuff. HyVis was apparently way ahead of the curve on extended oil use, because on the top of the can it says it’s good for 1,000 to 9,999 miles. Interestingly, the oil has zero additive in it (Figure 1). Or perhaps it’s got 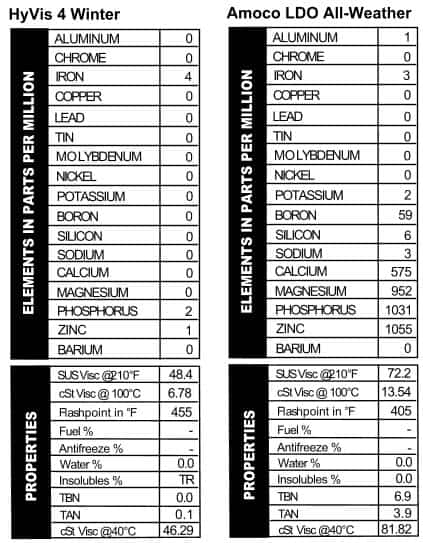 additive in it, just not any that we read. The grade is not listed on the can, but the viscosity came back as a light 20W. With the lack of additive it’s not surprising that the TBN read 0.0. It’s tempting to do an experiment and run this apparent mineral oil for 10,000 miles in the dead of winter, just to see what would happen. If you know of any guinea pigs, send them our way!
additive in it, just not any that we read. The grade is not listed on the can, but the viscosity came back as a light 20W. With the lack of additive it’s not surprising that the TBN read 0.0. It’s tempting to do an experiment and run this apparent mineral oil for 10,000 miles in the dead of winter, just to see what would happen. If you know of any guinea pigs, send them our way!
Amoco LDO 10W/40
Next up is a pair of old oils: Amoco and Texaco. These names remind me of gas stations we’d stop at on family vacations in the 1970s. Amoco (which he always pronounced Uh-muck-o) is the one that reminds me most of my Dad. Amoco gas stations were called Standard stations in some parts of  the country, and I’ll always associate the blue, red, and white logo with long trips across the country in our green van with the velour bed and hanging beads. But Amoco did more than fill up gas tanks in the 70s–they also sold oil, and this “Long Distance” version is an SAE 10W/40. The additive package looks a lot like the Mobil Special oil we saw: heavy on phosphorus and zinc, lighter on calcium and magnesium (Figure 2). Just the right oil for a couple of bandana-wearing hippies traveling with two little kids from Indiana to Nova Scotia in 1976 in a green van with a sunset painted on the side. Ah, the ’70s.
the country, and I’ll always associate the blue, red, and white logo with long trips across the country in our green van with the velour bed and hanging beads. But Amoco did more than fill up gas tanks in the 70s–they also sold oil, and this “Long Distance” version is an SAE 10W/40. The additive package looks a lot like the Mobil Special oil we saw: heavy on phosphorus and zinc, lighter on calcium and magnesium (Figure 2). Just the right oil for a couple of bandana-wearing hippies traveling with two little kids from Indiana to Nova Scotia in 1976 in a green van with a sunset painted on the side. Ah, the ’70s.
Texaco Ursa ED 20-20W and Texaco Havoline Super Premium 10W/40
The Texaco Ursa ED oil is scant on advertising copy. They must have had their hands full designing this “Extra Duty” 20-20W oil instead. On the can they recommend this oil for everything you can think of except lawn mowers. It’s got a lot of additive in it (Figure 3), but the additives are configured more like what we’d expect out of a gear lube ¾ except with more calcium. Why the 20-20W and not just 20W? We’re not sure. Maybe it’s fancier. 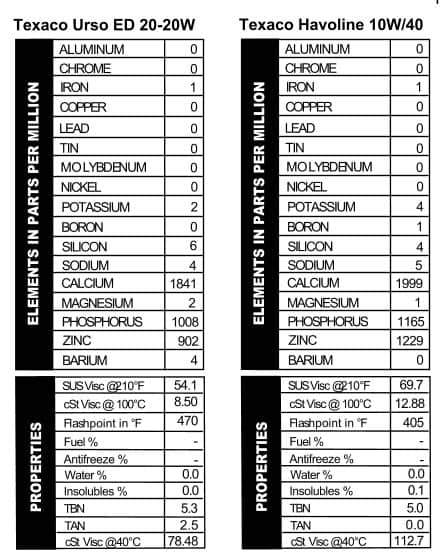 The viscosity read like what we see today out of a standard 5W/30. Texaco Havoline Super Premium 10W/40, on the other hand, looks a lot like one of today’s diesel-use oils in additives (Figure 4), with a normal 10W/40 viscosity.
The viscosity read like what we see today out of a standard 5W/30. Texaco Havoline Super Premium 10W/40, on the other hand, looks a lot like one of today’s diesel-use oils in additives (Figure 4), with a normal 10W/40 viscosity.
Lucky Strike 20-20W

We knew Lucky Strike made cigarettes, but we had no idea there was an oil of the same name until Ryan found this can and bought it on eBay for $29.99. The can looks seriously old, with no information on it beyond the name, a picture of an oil derrick, and the words “One U.S. Quart.” A quick Google search revealed no real information¾just some old Lucky Strike Oil & Gas Company stock certificates from 1917. The oil probably isn’t quite that old, but it may very well be the oldest of all the cans we bought. They didn’t do a lot with additives back then, though we did turn up 125 ppm barium (Figure 5). Not a lot else was present in this 20-20W oil. Note that without any calcium or magnesium, the TBN read 0.0.
Union 76 20W/50
Union 76 20W/50 is clearly made for speed. You can tell because of the black-and-white checkers on the front. According to the lid, this oil was tested and certified by the national association for stock car auto racing. My friend Google tells me that stock car racing became popular back in the 1920s (when moonshiners were outrunning Johnny Law during Prohibition times), but this can is clearly not that old. Not  only did they not make multi-viscosity oils that long ago, but the can comes from zip code 90017 so it has to be from 1963 or later. According to the can, it’s 100% parrafinic oil with selected additives, which our spectrometer reveals to be your standard line-up of calcium, phosphorus, and zinc (Figure 6). Look at that viscosity though¾it’s higher than we see in today’s 20W/50s.
only did they not make multi-viscosity oils that long ago, but the can comes from zip code 90017 so it has to be from 1963 or later. According to the can, it’s 100% parrafinic oil with selected additives, which our spectrometer reveals to be your standard line-up of calcium, phosphorus, and zinc (Figure 6). Look at that viscosity though¾it’s higher than we see in today’s 20W/50s. 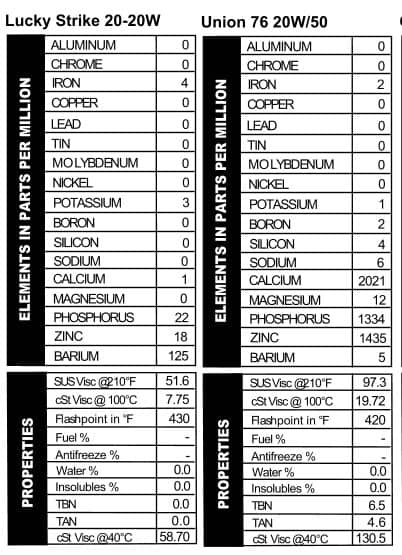
Castrol R Racing
Who doesn’t love Castrol? Other oil companies, that’s who. But we like them all right, so we bought two old cans. One, with liquid tungsten, we reviewed in the first article in this series. The other appears to be much older: I’d place it from the 1940s or 1950s. Not only does this can have a high opinion of itself (“The Masterpiece in Oils!”), but they direct you how to ask for it at the store: “Do not ask for ‘XL’ or ‘XXL.’ Always state the full name.”  Like modern oils, they stress the high quality of the oil with terms like “organo-metallic” that are meant impress those of us who aren’t in the oil business. I don’t know if I’d call this a masterpiece in oil work though: it looks like what we see out of ATFs these days as far as additives go (mostly phosphorus with a little zinc thrown in). Since the word “Racing” is stamped into the top of the can, the thick viscosity (like a 50W) makes sense (Figure 7).
Like modern oils, they stress the high quality of the oil with terms like “organo-metallic” that are meant impress those of us who aren’t in the oil business. I don’t know if I’d call this a masterpiece in oil work though: it looks like what we see out of ATFs these days as far as additives go (mostly phosphorus with a little zinc thrown in). Since the word “Racing” is stamped into the top of the can, the thick viscosity (like a 50W) makes sense (Figure 7).
Sinclair Dinolene 20-20W
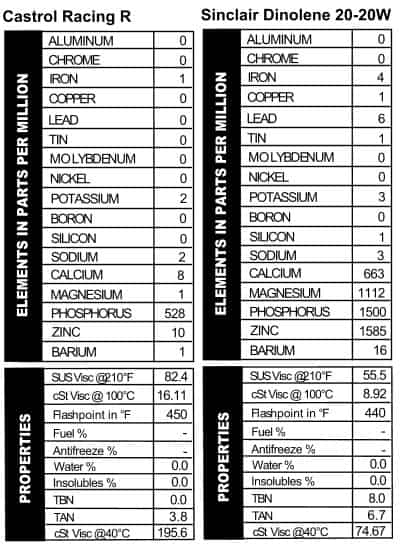 I’ve always had a soft spot in my heart for Sinclair. The can has a picture of a dinosaur on it! This shit came from the ground, no doubt about it! Another 20-20W oil, the oil is light on advertising copy but heavy on additives. In fact, it looks a lot like recent versions of Shell’s Rotella 5W/40, except with a little less calcium. It’s much lighter than Shell’s 5W/40, though, with a viscosity reading like a 30W or a heavy 20W oil. Note the presence of lead (Figure 8).
I’ve always had a soft spot in my heart for Sinclair. The can has a picture of a dinosaur on it! This shit came from the ground, no doubt about it! Another 20-20W oil, the oil is light on advertising copy but heavy on additives. In fact, it looks a lot like recent versions of Shell’s Rotella 5W/40, except with a little less calcium. It’s much lighter than Shell’s 5W/40, though, with a viscosity reading like a 30W or a heavy 20W oil. Note the presence of lead (Figure 8). 
Amsoil Super Premium 10W/40
Until I started writing this article, I had no idea that Amsoil has been around since 1972. This particular sample came, like all the rest, from a can, so it has to be at least from the mid-’80s (which is when most companies switched to plastic bottles). Even 30+ years ago, Amsoil was pushing 25,000-mile oil changes, and the can even lists a comparison between this oil and regular old petroleum as far as engine temps, flashpoint, oxidation, and lubrication range (unsurprisingly, Amsoil wins in each category!).  Modern Amsoil products tend to be heavy on additives and that was true back in the day as well (Figure 9). Just for fun, we compared this oil with a virgin sample of Amsoil 10W/40 that we ran in February 2012 and they look a lot alike. The only difference is in the older oil there’s less of everything: 500 ppm less calcium, and 100-200 ppm less phosphorus and zinc. They used magnesium in the older oil, while the TBN and viscosities were nearly identical.
Modern Amsoil products tend to be heavy on additives and that was true back in the day as well (Figure 9). Just for fun, we compared this oil with a virgin sample of Amsoil 10W/40 that we ran in February 2012 and they look a lot alike. The only difference is in the older oil there’s less of everything: 500 ppm less calcium, and 100-200 ppm less phosphorus and zinc. They used magnesium in the older oil, while the TBN and viscosities were nearly identical.
Pennzoil Dex2 and Z-7 10W/40
Pennzoil started as Penn’s Oil in 1913, and I have to admit, “Why the Liberty Bell?” was my most pressing question as I looked 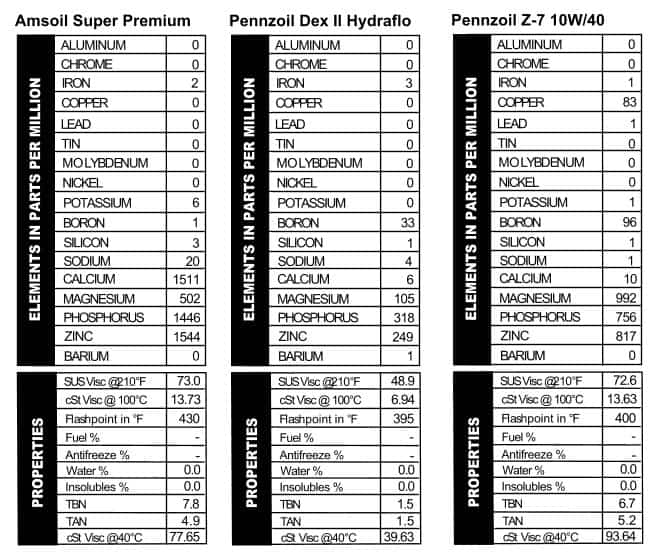 over these two old cans. Originally, Penn’s Oil came from an oil field in Pennsylvania and was christened with a Liberty Bell logo to remind users of its Pennsylvania roots. This can of ATF clearly has an earlier generation of logo on it, and Google informs me that Dexron II was introduced in 1972. This may very well have been the transmission oil that kept our green van chugging through the ’70s. There are a lot of different ATFs in stores today, though generally they have about the same additive configurations. This one is a little different in that it has more boron, magnesium, and zinc than most modern ATFs. The viscosity is right where we’d expect it to be though. Pennzoil’s 10W/40 oil can is flashy, a la the
over these two old cans. Originally, Penn’s Oil came from an oil field in Pennsylvania and was christened with a Liberty Bell logo to remind users of its Pennsylvania roots. This can of ATF clearly has an earlier generation of logo on it, and Google informs me that Dexron II was introduced in 1972. This may very well have been the transmission oil that kept our green van chugging through the ’70s. There are a lot of different ATFs in stores today, though generally they have about the same additive configurations. This one is a little different in that it has more boron, magnesium, and zinc than most modern ATFs. The viscosity is right where we’d expect it to be though. Pennzoil’s 10W/40 oil can is flashy, a la the  1980s. It’s “The Motor Oil With Z-7” and although they don’t specify what that is, they do specify that “You need no extra oil additive.” So that’s reassuring. It’s rated SF-SC-CC, so I’d place it at about 25 years old. Maybe the magic of Z-7 is copper: that’s something we saw a lot of back in the day, when Blackstone was founded. Interestingly, magnesium is the dominant additive in this one, followed by zinc, phosphorus, copper, and boron (Figure 10). The flashpoint was lower than what we see today from 10W/40s.
1980s. It’s “The Motor Oil With Z-7” and although they don’t specify what that is, they do specify that “You need no extra oil additive.” So that’s reassuring. It’s rated SF-SC-CC, so I’d place it at about 25 years old. Maybe the magic of Z-7 is copper: that’s something we saw a lot of back in the day, when Blackstone was founded. Interestingly, magnesium is the dominant additive in this one, followed by zinc, phosphorus, copper, and boron (Figure 10). The flashpoint was lower than what we see today from 10W/40s. 
Renuzit
At last, we finish our tour of ancient oils. (Some of these samples are older than the people reading this article. This makes me feel very old. Hey, get off my lawn!) I said before that I thought the Lucky Strike was the oldest of the oils we tested, but that’s only because I forgot about this can of Renuzit sitting in Blackstone’s garage. It’s rusty on the bottom so it leaks, and it’s not from 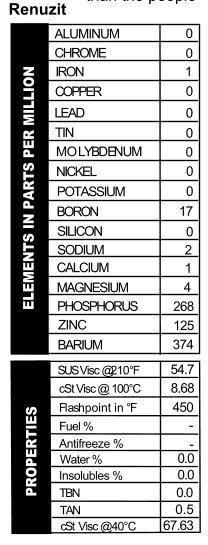 a can like the others. This one was sold in a 5-quart metal jug. I particularly love this one, because the can not only says you’ll “Cut Your Oil Bills In Half,” but the first point of advertising on the side is a “Faster Getaway!” Now, they don’t actually say that this is the choice of oils for bank robbers, but I know if it was 1941 and my hungry Great Depression self was contemplating which oil to put in my Ford Special for bank-robbing time, this is the oil I’d pick. With practically no calcium or magnesium present, the oil’s TBN read 0.0, but it does provide would-be crooks with phosphorus, zinc, and barium as well as a 20W viscosity for the getaway (Figure 11). When you’re busy working a tommy gun, the last think you want to think about is whether or not you’ve made the right choice in oil.
a can like the others. This one was sold in a 5-quart metal jug. I particularly love this one, because the can not only says you’ll “Cut Your Oil Bills In Half,” but the first point of advertising on the side is a “Faster Getaway!” Now, they don’t actually say that this is the choice of oils for bank robbers, but I know if it was 1941 and my hungry Great Depression self was contemplating which oil to put in my Ford Special for bank-robbing time, this is the oil I’d pick. With practically no calcium or magnesium present, the oil’s TBN read 0.0, but it does provide would-be crooks with phosphorus, zinc, and barium as well as a 20W viscosity for the getaway (Figure 11). When you’re busy working a tommy gun, the last think you want to think about is whether or not you’ve made the right choice in oil.
So that’s the end of our series. A lot of thought goes into making oil, and that’s been the case for many decades now. We’ve poked some fun at the way oil companies sell their products, but heck, they’ve got to say something. We stand by our statement that “oil is oil” and in the end, it doesn’t make a lot of difference what you decide to use. Through all the years and all the permutations and configurations of oil and oil additives, the crude (and now, synthetic) stuff has kept cars running since Henry Ford did his thing more than 100 years ago. Buy what suits your vehicle and your wallet¾not what anyone else says you should use!